Electrons Have A ______ Charge.
cibeltiagestion
Sep 11, 2025 · 7 min read
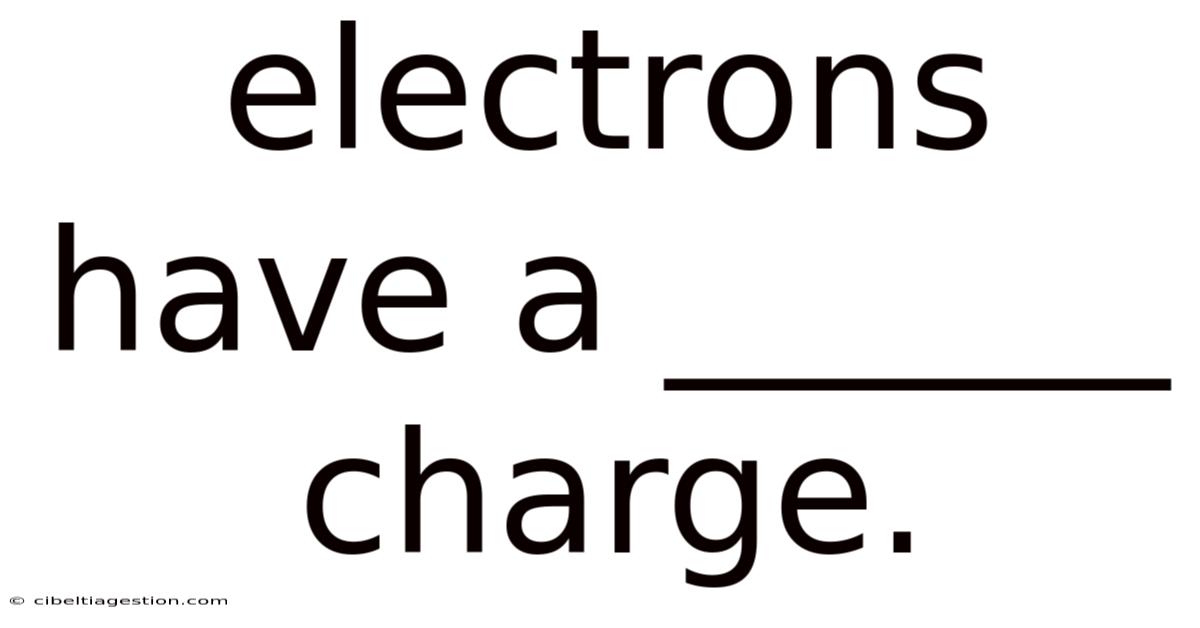
Table of Contents
Electrons Have a Negative Charge: A Deep Dive into the Subatomic World
The simple statement, "electrons have a negative charge," underpins our understanding of electricity, chemistry, and the very fabric of matter. This seemingly straightforward fact opens a door to a fascinating world of subatomic particles, their interactions, and the profound implications for everything around us. This article will explore this fundamental concept in depth, examining not just the what but also the why and how of electron charge. We'll delve into the historical context of its discovery, the experimental evidence supporting it, and its broader significance in science and technology.
Introduction: A Brief History of the Electron's Charge
The journey to understanding the negative charge of electrons is a fascinating chapter in the history of science. While the concept of electricity was known for centuries, the understanding of its fundamental nature remained elusive until the late 19th and early 20th centuries. Scientists like Michael Faraday made significant contributions by studying electrolysis and demonstrating the relationship between electricity and chemical reactions. However, it wasn't until J.J. Thomson's experiments with cathode ray tubes in 1897 that the electron was definitively identified as a fundamental particle carrying a negative charge.
Thomson's experiments showed that cathode rays, streams of particles emitted from a cathode in a vacuum tube, were deflected by both electric and magnetic fields. The direction of deflection indicated that these particles carried a negative charge. He meticulously measured the charge-to-mass ratio (e/m) of these particles, finding it to be significantly larger than that of any known ion, suggesting a much smaller mass. This groundbreaking work paved the way for the acceptance of the electron as a fundamental constituent of matter. Further experiments by Robert Millikan in his famous oil-drop experiment determined the magnitude of the electron's charge, solidifying the understanding of its negative nature.
Understanding the Magnitude of the Charge
The electron's charge is often represented by the symbol 'e' and has a value of approximately -1.602 x 10⁻¹⁹ Coulombs. This minuscule value highlights the incredible sensitivity of our instruments that can detect and measure such a tiny charge. The Coulomb (C) is the standard unit of electric charge in the International System of Units (SI). It's important to note that the negative sign explicitly signifies the negative nature of the electron's charge, distinguishing it from the positive charge of the proton.
This negative charge is a fundamental property of the electron, meaning it's an intrinsic characteristic that cannot be further subdivided or explained by a simpler underlying mechanism. It's a defining feature of the electron, just as its mass and spin are. The exact mechanism behind the electron's charge remains a topic of ongoing research within the realm of quantum field theory.
The Role of Charge in Atomic Structure
The electron's negative charge plays a crucial role in defining the structure of atoms. Atoms consist of a positively charged nucleus containing protons and neutrons, surrounded by a cloud of negatively charged electrons. The electrostatic attraction between the positively charged nucleus and the negatively charged electrons holds the atom together. The number of protons in the nucleus determines the element, while the number of electrons determines the atom's overall charge. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero. However, atoms can gain or lose electrons, forming ions with a net positive (cation) or negative (anion) charge.
The arrangement of electrons in an atom, governed by quantum mechanics, determines the atom's chemical properties and how it interacts with other atoms. Electrons occupy specific energy levels or orbitals around the nucleus, and their interactions dictate the formation of chemical bonds and the creation of molecules. The study of electron configuration and its implications for chemical bonding is a cornerstone of chemistry.
Experimental Evidence: Beyond Cathode Rays
While Thomson's cathode ray experiments provided the initial evidence for the electron's negative charge, numerous other experiments have since corroborated this finding. These include:
-
Millikan's Oil Drop Experiment: This experiment precisely measured the charge of a single electron by observing the motion of charged oil droplets in an electric field. The results confirmed the quantized nature of electric charge and provided a precise value for the elementary charge, 'e'.
-
Spectroscopy: The analysis of light emitted or absorbed by atoms reveals the energy levels of electrons within the atom. The specific wavelengths of light correspond to transitions of electrons between different energy levels, providing indirect but strong evidence for the existence and behavior of negatively charged electrons.
-
Photoelectric Effect: Einstein's explanation of the photoelectric effect demonstrated that light can eject electrons from a metal surface. The energy of the ejected electrons depends on the frequency of the light, further confirming the existence of electrons and their interaction with electromagnetic radiation.
Implications of Electron Charge in Everyday Life
The negative charge of the electron is not just a scientific curiosity; it underpins many technologies and phenomena we encounter daily:
-
Electricity: The flow of electrons in a conductor constitutes an electric current, the basis of our electrical power grid, electronic devices, and countless other applications.
-
Chemistry: Chemical reactions are driven by the interactions between electrons in atoms and molecules. The electron's charge determines the type and strength of chemical bonds, impacting the properties of substances and the reactions they undergo.
-
Electronics: Transistors, integrated circuits, and other electronic components rely on the controlled flow of electrons to process information and perform complex operations.
-
Medical Imaging: Techniques like X-rays and computed tomography (CT) scans utilize the interaction of electrons with electromagnetic radiation to create images of the internal structures of the body.
Quantum Mechanics and the Electron's Charge
The electron's charge takes on even more profound significance when viewed through the lens of quantum mechanics. In the quantum world, the electron's charge is not just a property; it is a fundamental quantum number, an intrinsic characteristic that determines its interactions with electromagnetic fields. Quantum electrodynamics (QED) describes the interaction of electrons with photons, the particles of light, with remarkable accuracy. This theory explains the electron's behavior in terms of probability and wave functions, providing a deeper understanding of its charge and its interactions with other particles.
The concept of quantization, where charge exists in discrete units (multiples of 'e'), is a core principle of quantum mechanics. This means that you cannot have half an electron's charge or any fractional value; it always exists as a whole number multiple of 'e'.
Frequently Asked Questions (FAQ)
Q: Can an electron lose its charge?
A: No, the electron's charge is a fundamental property and cannot be lost. It's an intrinsic characteristic, much like its mass or spin.
Q: What is the difference between an electron and a positron?
A: An electron is a negatively charged lepton, while a positron is its antiparticle, carrying the same mass but a positive charge. When an electron and a positron collide, they annihilate each other, converting their mass into energy in the form of photons.
Q: How is the electron's charge measured?
A: The electron's charge is not directly measured with a simple instrument like a ruler. Sophisticated techniques, like Millikan's oil-drop experiment or modern variations of it, are used to indirectly determine its value by observing the effect of its electric field on other particles.
Q: Why is the electron's charge negative?
A: The reason for the negative charge of the electron is still a subject of fundamental research in physics. It's considered a fundamental property, a building block of nature, without a deeper mechanistic explanation at this time.
Conclusion: A Fundamental Constant Shaping Our World
The simple statement, "electrons have a negative charge," encapsulates a vast body of knowledge and countless scientific advancements. This fundamental property, revealed through meticulous experimentation and elegant theoretical frameworks, underpins our understanding of the universe at its most basic level. From the structure of atoms to the functioning of electronic devices, the negative charge of the electron profoundly influences our world, shaping the technology we use and the reality we experience. Continued research into the electron and its properties will undoubtedly uncover even deeper insights into the fundamental laws of nature and the intricate workings of our universe. Understanding the electron's negative charge is not just about memorizing a fact; it is about grasping a pivotal concept that unlocks a deeper appreciation for the complexity and elegance of the natural world.
Latest Posts
Latest Posts
-
What Is 80 Of 150
Sep 11, 2025
-
Sudden Wind Gusts On Highways
Sep 11, 2025
-
What Does 12 6 Sign Mean
Sep 11, 2025
-
Right Foot Amputation Icd 10
Sep 11, 2025
-
Which Scatterplot Shows No Correlation
Sep 11, 2025
Related Post
Thank you for visiting our website which covers about Electrons Have A ______ Charge. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.