Enantiomers Are Molecules That _____.
cibeltiagestion
Sep 16, 2025 · 6 min read
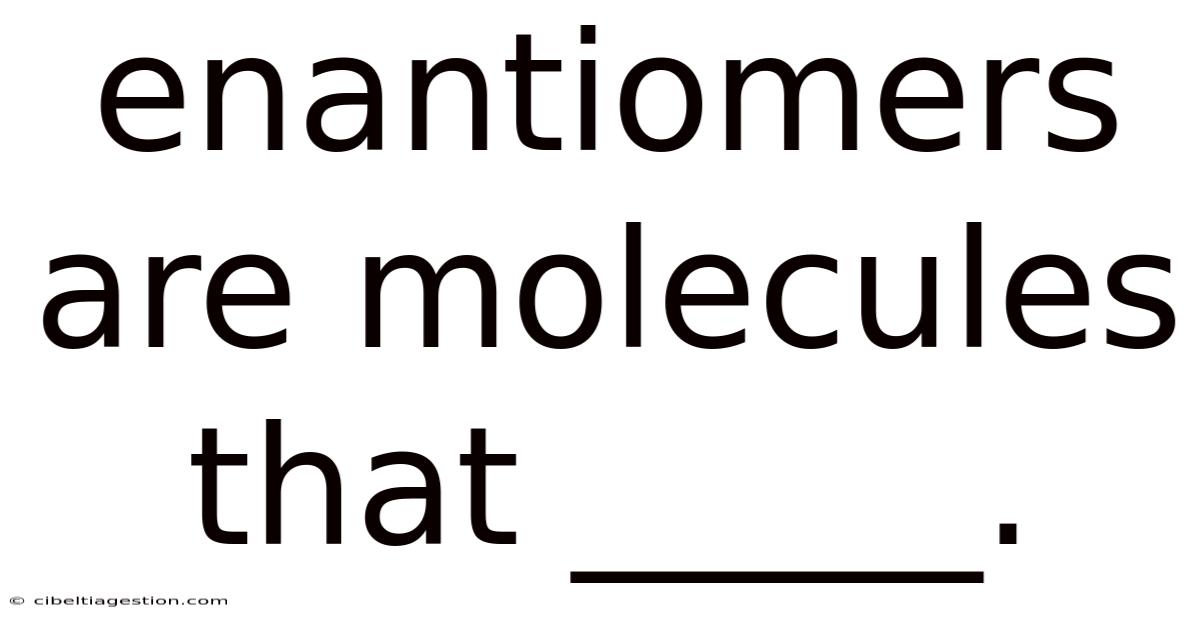
Table of Contents
Enantiomers Are Molecules That… Are Mirror Images of Each Other
Enantiomers are molecules that are non-superimposable mirror images of each other. This seemingly simple definition belies a profound impact on chemistry, biology, and medicine. Understanding enantiomers requires delving into the fascinating world of chirality, stereochemistry, and the subtle yet significant differences between seemingly identical molecules. This detailed exploration will illuminate the concept of enantiomers, their properties, identification methods, and their critical role in various fields.
Introduction to Chirality and Stereochemistry
Before diving into the intricacies of enantiomers, we need to establish a foundational understanding of chirality and stereochemistry. Chirality refers to the property of a molecule that cannot be superimposed on its mirror image. Imagine holding your left hand up against a mirror; the reflection is identical to your right hand, but you cannot overlay your left hand perfectly onto the reflection of your right hand. Similarly, chiral molecules exist as two non-superimposable mirror images. These mirror images are called enantiomers.
Stereochemistry is the branch of chemistry that deals with the three-dimensional arrangement of atoms in molecules and how this arrangement affects their properties. Enantiomers are a key aspect of stereochemistry, highlighting the importance of spatial arrangement in determining molecular behavior. A molecule's stereochemistry significantly impacts its reactivity, physical properties (like melting point and optical rotation), and biological activity.
Defining Enantiomers: More Than Just Mirror Images
While the mirror image description is a good starting point, it's crucial to emphasize that enantiomers are non-superimposable mirror images. This means you cannot rotate the molecule in three-dimensional space and perfectly align it with its mirror image. This non-superimposability is a direct consequence of the presence of at least one chiral center within the molecule.
A chiral center (also known as a stereocenter or asymmetric carbon) is typically a carbon atom bonded to four different groups. This tetrahedral arrangement creates a spatial asymmetry that leads to chirality. Molecules without chiral centers are achiral, meaning they are superimposable on their mirror images. The presence of multiple chiral centers within a molecule increases the number of possible stereoisomers, which can include enantiomers and diastereomers (stereoisomers that are not mirror images).
Properties of Enantiomers: A Tale of Two Molecules
Despite their identical chemical formulas and connectivity, enantiomers exhibit distinct properties in certain contexts. These differences arise from their different three-dimensional arrangements:
-
Optical Activity: This is perhaps the most well-known difference. Enantiomers rotate plane-polarized light in opposite directions. One enantiomer rotates the light clockwise (dextrorotatory, denoted as + or d), while the other rotates it counterclockwise (levorotatory, denoted as – or l). The magnitude of rotation is the same for both enantiomers, but the direction is opposite. This property is measured using a polarimeter.
-
Physical Properties: In many cases, enantiomers have identical physical properties such as melting point, boiling point, and solubility in achiral solvents. However, they can exhibit different physical properties when interacting with chiral environments, such as chiral solvents or crystals.
-
Chemical Properties: Enantiomers react identically with achiral reagents. However, their reactivity differs significantly when interacting with chiral reagents or catalysts. This selective reactivity is crucial in many biological processes.
-
Biological Activity: This is where the profound impact of enantiomers becomes most evident. Enzymes, being chiral molecules themselves, often exhibit remarkable stereoselectivity, interacting differently with each enantiomer. One enantiomer may be biologically active, while its mirror image is inactive or even toxic. This is a critical consideration in drug design and development.
Naming and Representing Enantiomers: The R/S System and Fischer Projections
Several systems are used to name and represent enantiomers. The most widely used is the Cahn-Ingold-Prelog (CIP) system, which assigns priorities to the four groups attached to the chiral center based on atomic number. This leads to the designation of R (rectus, Latin for right) or S (sinister, Latin for left) for each enantiomer.
Fischer projections are a simplified two-dimensional representation of three-dimensional molecules. They are particularly useful for depicting chiral centers and visualizing the relationship between enantiomers. However, it's essential to understand the limitations of Fischer projections and their potential for misinterpretation.
Methods for Separating Enantiomers: Resolution
Separating enantiomers, a process called resolution, is a significant challenge in chemistry due to their nearly identical physical properties. Several techniques are employed:
-
Chiral Chromatography: This method uses a stationary phase that interacts differently with each enantiomer, leading to their separation based on their differential retention times.
-
Diastereomer Formation: Enantiomers can be converted into diastereomers through reaction with a chiral reagent. Diastereomers have different physical properties and can be separated using conventional techniques like crystallization or distillation. The diastereomers are then converted back to the pure enantiomers.
-
Enzymatic Resolution: Enzymes, being highly stereoselective, can be used to selectively react with one enantiomer, leaving the other enantiomer untouched. This approach is particularly useful for separating enantiomers of biologically important molecules.
Enantiomers in the Real World: Biological Activity and Drug Design
The significance of enantiomers extends far beyond academic research. Their distinct biological activities have profound implications across diverse fields:
-
Pharmaceuticals: Many drugs exist as enantiomers, and often only one enantiomer is responsible for the therapeutic effect. The other enantiomer may be inactive or even cause adverse effects. Developing enantiomerically pure drugs is, therefore, a crucial aspect of modern pharmaceutical research. Thalidomide, a tragic example, illustrates the devastating consequences of using a drug mixture containing both enantiomers with differing biological activities.
-
Pesticides and Herbicides: Similar to pharmaceuticals, the effectiveness and environmental impact of pesticides and herbicides are greatly influenced by enantiomeric purity.
-
Flavor and Fragrance: Many natural products responsible for flavors and fragrances exist as enantiomers, and their odor and taste can differ significantly.
-
Food Science: Enantiomeric purity is essential in food science and nutrition, influencing the bioavailability and metabolic fate of food components.
Frequently Asked Questions (FAQs)
-
Q: Can I tell the difference between enantiomers just by looking at their chemical formulas? A: No. Chemical formulas only show the connectivity of atoms, not their three-dimensional arrangement. You need to consider the spatial arrangement to differentiate enantiomers.
-
Q: Are all molecules with chiral centers chiral? A: No. Some molecules with chiral centers possess internal symmetry that cancels out the chirality, making them achiral (meso compounds).
-
Q: Why is separating enantiomers challenging? A: Enantiomers have nearly identical physical properties, making conventional separation techniques ineffective. Specialized methods are required.
-
Q: What happens if a drug is administered as a racemic mixture (a 50:50 mixture of both enantiomers)? A: The effects depend on the biological activity of each enantiomer. One enantiomer might be therapeutic, while the other is inactive or harmful.
-
Q: What is the importance of enantiomerically pure compounds? A: Enantiomerically pure compounds are crucial in many fields, particularly pharmaceuticals, where the undesired enantiomer might have adverse effects or no therapeutic value.
Conclusion: The Enantiomeric World
Enantiomers, while seemingly subtle variations in molecular structure, possess profoundly different properties and biological activities. Their study underscores the critical importance of stereochemistry in understanding molecular behavior and designing molecules with specific properties. From drug development to environmental science, the consideration of enantiomers is crucial for advancing scientific knowledge and improving human life. The journey into the world of enantiomers is a fascinating exploration into the intricate relationship between structure and function in the molecular world, a world where even the slightest change in spatial arrangement can have dramatic consequences.
Latest Posts
Latest Posts
-
How Fast Lion Can Run
Sep 16, 2025
-
70 F Convert To Celsius
Sep 16, 2025
-
Factor X 2 4x 5
Sep 16, 2025
-
A992 Steel Modulus Of Elasticity
Sep 16, 2025
-
128 Ounces In A Gallon
Sep 16, 2025
Related Post
Thank you for visiting our website which covers about Enantiomers Are Molecules That _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.