Full Electron Configuration For Pb
cibeltiagestion
Sep 11, 2025 · 6 min read
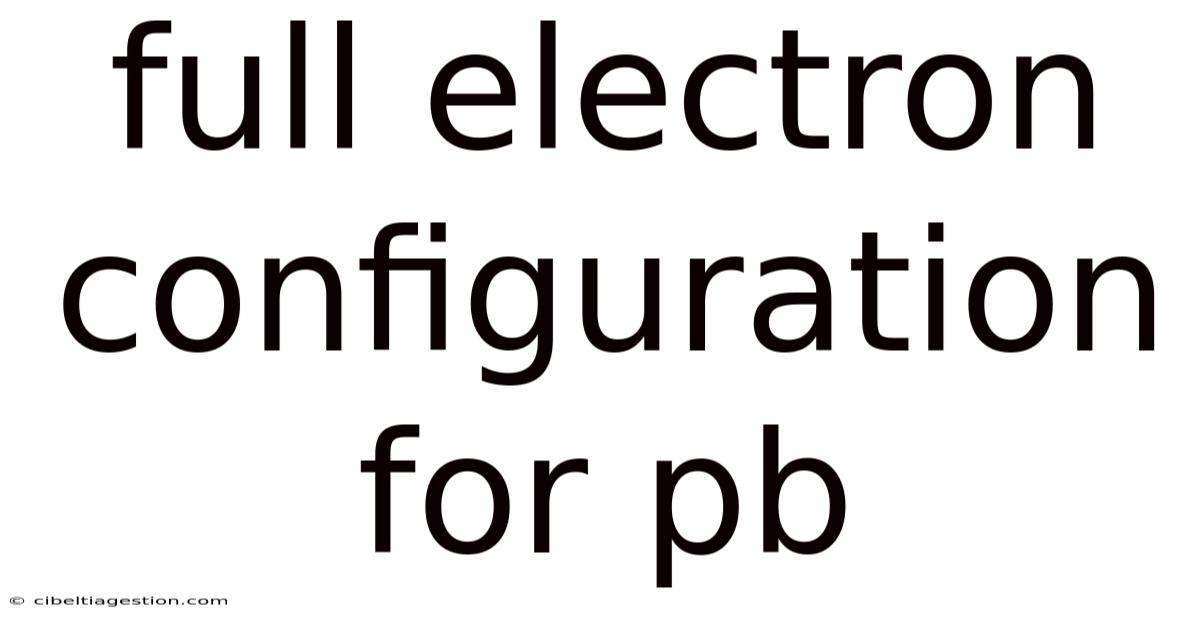
Table of Contents
Unveiling the Secrets of Lead: A Deep Dive into the Full Electron Configuration of Pb
Lead (Pb), a heavy metal with a rich history, finds applications ranging from ancient Roman plumbing to modern-day batteries. Understanding its properties requires delving into its atomic structure, specifically its electron configuration. This article will provide a comprehensive explanation of the full electron configuration of lead, exploring its underlying principles, implications, and connections to its chemical behavior. We will also examine related concepts to solidify your understanding of this fascinating element.
Introduction: Deciphering the Atomic Structure
The electron configuration of an atom describes how electrons are distributed among its various energy levels and sublevels. This arrangement dictates an element's chemical properties, reactivity, and bonding behavior. Lead, with its atomic number of 82, possesses 82 electrons that need to be systematically placed within its orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle. Understanding these fundamental principles is crucial before we dive into the specifics of lead's electron configuration.
The Aufbau Principle and Electron Filling
The Aufbau principle dictates that electrons fill atomic orbitals in order of increasing energy levels. This means that lower energy levels are filled before higher energy levels. The order of filling can be summarized using the mnemonic device, "1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p..." Each orbital can hold a maximum of two electrons, as stated by the Pauli exclusion principle.
Hund's Rule and Orbital Stability
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the electrons, leading to a more stable configuration. This rule is particularly important when dealing with p, d, and f subshells, which contain multiple orbitals.
The Pauli Exclusion Principle: A Fundamental Rule
The Pauli exclusion principle is a cornerstone of quantum mechanics. It states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can only hold a maximum of two electrons, and these two electrons must have opposite spins (spin up and spin down).
Deriving the Full Electron Configuration of Lead (Pb)
Now, let's apply these principles to determine the full electron configuration of lead (Pb), which has 82 electrons. Following the Aufbau principle, we fill the orbitals sequentially:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p²
This configuration can also be written in a condensed or shorthand notation using the noble gas configuration:
[Xe] 4f¹⁴ 5d¹⁰ 6s² 6p²
Here, [Xe] represents the electron configuration of Xenon (Xe), the noble gas preceding lead in the periodic table. This shorthand notation simplifies the representation while still conveying all the essential information about the electron distribution.
Explanation of the Electron Configuration:
Let's break down the significance of each part of the lead's electron configuration:
-
[Xe]: This represents the core electrons, essentially the electron configuration of Xenon (54 electrons). These inner electrons are tightly bound to the nucleus and generally do not participate in chemical bonding.
-
4f¹⁴: The fourteen electrons in the 4f subshell are characteristic of the lanthanides. This subshell is relatively shielded from external influences, reducing its impact on lead's chemical reactivity.
-
5d¹⁰: The ten electrons in the 5d subshell contribute to lead's metallic character and its relatively high density.
-
6s²: The two electrons in the 6s subshell are relatively high in energy and are more readily involved in chemical bonding.
-
6p²: The two electrons in the 6p subshell are the valence electrons – the outermost electrons that are primarily responsible for lead's chemical behavior and reactivity. These electrons are easily lost or shared during chemical reactions.
Implications of Lead's Electron Configuration:
Lead's electron configuration explains several of its key properties:
-
Metallic Character: The presence of numerous electrons in the d and f subshells contributes significantly to lead's metallic character, including its luster, conductivity, and malleability.
-
Low Reactivity: While lead is a metal, it exhibits relatively low chemical reactivity compared to other metals in its group (Group 14). This is partly due to the relatively poor shielding of the outer electrons by the inner electrons, resulting in a higher effective nuclear charge that holds the valence electrons more tightly.
-
Variable Oxidation States: Lead exhibits variable oxidation states, primarily +2 and +4. The +2 oxidation state arises from the loss of the two 6p electrons, while the +4 oxidation state involves the loss of both the 6p and 6s electrons. This variability is reflected in the diverse range of lead compounds.
-
Toxicity: Lead's toxicity is a well-known and concerning aspect of its properties. While the exact mechanisms are complex and still under investigation, the electronic structure plays a role in its ability to interact with biological molecules, potentially disrupting cellular processes and causing various health problems.
Frequently Asked Questions (FAQ)
-
Q: What is the difference between the full and condensed electron configuration?
A: The full electron configuration shows the electron occupancy of every subshell from 1s onwards. The condensed configuration uses the noble gas configuration as a shorthand to represent the core electrons, simplifying the representation.
-
Q: Why is the order of filling orbitals not strictly sequential in terms of principal quantum number (n)?
A: The energy levels of orbitals are not solely determined by the principal quantum number. Shielding effects and electron-electron repulsions influence the energy levels, leading to deviations from a purely sequential filling order (for instance, the 4s orbital fills before the 3d orbital).
-
Q: How does the electron configuration relate to lead's position in the periodic table?
A: Lead's position in the periodic table (Group 14, Period 6) directly reflects its electron configuration. Its placement in Group 14 indicates two valence electrons in the p-subshell, and its position in Period 6 indicates six principal energy levels containing electrons.
-
Q: Can the electron configuration predict all the properties of lead?
A: While the electron configuration provides a foundation for understanding lead's chemical behavior, it doesn't predict all properties. Other factors like intermolecular forces, crystal structure, and isotopic composition also play crucial roles.
Conclusion: A Deeper Appreciation of Lead
Understanding the full electron configuration of lead is not merely an academic exercise; it's a key to understanding its properties, reactivity, and applications. By applying the fundamental principles of atomic structure, we've successfully determined and interpreted lead's electron configuration. This knowledge helps us appreciate the complex interplay between electronic structure and the physical and chemical characteristics of this fascinating and historically significant element. Further exploration of quantum mechanics and advanced chemical principles will provide an even more comprehensive understanding of lead and its unique properties. Remember, understanding the fundamental principles opens doors to understanding the intricacies of the world around us.
Latest Posts
Latest Posts
-
What Is 40 Of 135
Sep 11, 2025
-
Industrialization Influences Rates Of Literacy
Sep 11, 2025
-
Blue Gray Text 2 Excel
Sep 11, 2025
-
What Is 30 Off 60
Sep 11, 2025
-
1935 Two Dollar Bill Worth
Sep 11, 2025
Related Post
Thank you for visiting our website which covers about Full Electron Configuration For Pb . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.