Lewis Dot Structure For Xecl2
cibeltiagestion
Sep 15, 2025 · 7 min read
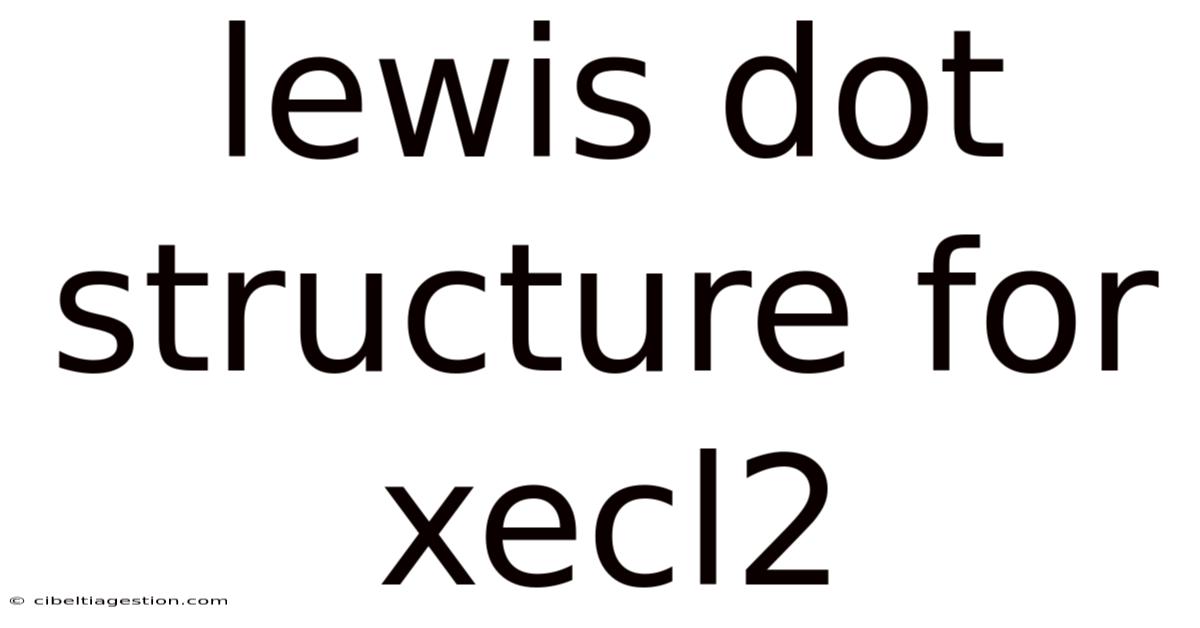
Table of Contents
Unveiling the Lewis Dot Structure of XeCl₂: A Deep Dive into Xenon's Unexpected Chemistry
Understanding the Lewis dot structure of a molecule is fundamental to grasping its bonding, shape, and overall properties. This article delves into the fascinating case of XeCl₂, xenon dichloride, a compound that challenges traditional chemical understanding and provides a valuable example of expanded octet structures. We will explore its Lewis structure step-by-step, discuss the underlying principles, and address frequently asked questions. This detailed explanation will help you confidently construct and interpret Lewis structures for similar molecules involving noble gases.
Introduction: Challenging the Inertness of Noble Gases
For decades, noble gases like xenon were considered inert, incapable of forming chemical bonds. Their electron configuration, with a full valence shell of eight electrons (octet), seemingly precluded any reactivity. However, Neil Bartlett's groundbreaking work in the 1960s demonstrated that this assumption was not entirely accurate. He synthesized the first noble gas compound, xenon hexafluoroplatinate (XePtF₆), proving that under specific conditions, noble gases can participate in chemical bonding. XeCl₂, one such compound, provides a compelling case study for understanding expanded octets and the nuances of Lewis structure representation.
Step-by-Step Construction of the Lewis Dot Structure for XeCl₂
Constructing the Lewis dot structure for XeCl₂ involves a systematic approach:
-
Determine the total number of valence electrons: Xenon (Xe) is in Group 18 and has 8 valence electrons. Chlorine (Cl) is in Group 17 and has 7 valence electrons. Since we have two chlorine atoms, the total number of valence electrons is 8 + 7 + 7 = 22.
-
Identify the central atom: Xenon, being less electronegative than chlorine, acts as the central atom.
-
Connect the atoms with single bonds: Connect the two chlorine atoms to the central xenon atom using single bonds. Each single bond consists of two electrons, so we've used 4 electrons (2 bonds x 2 electrons/bond).
-
Distribute remaining electrons to satisfy the octet rule (initially): We have 18 electrons remaining (22 - 4). We start by completing the octets of the chlorine atoms. Each chlorine atom needs 6 more electrons to achieve a stable octet (8 electrons). This uses 12 electrons (2 Cl atoms x 6 electrons/atom).
-
Handle the central atom: After satisfying the chlorine octets, we have 6 electrons remaining. These are placed on the xenon atom as lone pairs.
-
Assess for expanded octets: Notice that the xenon atom now has 12 electrons surrounding it (2 from each bond and 6 from the lone pairs). This is an expanded octet, exceeding the typical octet rule. This is permissible for elements in the third period (and beyond) due to the availability of vacant d orbitals. These orbitals can participate in bonding, allowing for more than eight electrons around the central atom.
-
Final Lewis Dot Structure: The final Lewis dot structure of XeCl₂ shows xenon in the center, bonded to two chlorine atoms with single bonds and three lone pairs of electrons around the xenon atom. Each chlorine atom has three lone pairs.
..
:Cl:
..
|
:Xe:
|
:Cl:
..
Understanding the Bonding in XeCl₂: Beyond the Octet Rule
The formation of XeCl₂ demonstrates an exception to the octet rule, a guideline that simplifies bonding descriptions for many elements. The octet rule stems from the stability associated with a filled valence shell (eight electrons). However, elements in the third period and beyond possess empty d orbitals that can participate in bonding. These d orbitals allow for the accommodation of more than eight electrons around the central atom, leading to hypervalent molecules like XeCl₂.
The bonding in XeCl₂ involves the overlap of the xenon's filled 5p orbitals with the chlorine's half-filled 3p orbitals. This leads to the formation of two covalent bonds, each involving a shared pair of electrons. The three lone pairs on the xenon atom occupy the remaining 5p and 5d orbitals.
Molecular Geometry and Hybridization: Predicting the Shape
The VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict the molecular geometry of XeCl₂. VSEPR suggests that electron pairs, whether bonding or non-bonding (lone pairs), repel each other and arrange themselves to minimize repulsion. In XeCl₂, the central xenon atom is surrounded by five electron pairs: two bonding pairs and three lone pairs.
According to VSEPR, the arrangement of five electron pairs leads to a trigonal bipyramidal electron geometry. However, the molecular geometry (considering only the atom positions) is linear, because the bonding pairs are arranged 180° apart, while the lone pairs occupy equatorial positions.
The hybridization of the xenon atom in XeCl₂ can be described as sp₃d, where one s, three p, and one d orbital hybridize to form five hybrid orbitals. These hybrid orbitals are used to accommodate the two bonding pairs and three lone pairs.
Importance and Applications of XeCl₂ and Related Compounds
Although XeCl₂ is not widely used in everyday applications, its existence is crucial for several reasons:
-
Expanding chemical understanding: The synthesis and study of XeCl₂ and other noble gas compounds significantly broadened our understanding of chemical bonding and the reactivity of elements previously considered inert. It challenged established paradigms and spurred research into new areas of chemistry.
-
Theoretical advancements: Investigating the bonding and structure of XeCl₂ has led to advancements in theoretical chemistry, particularly in the area of computational chemistry and density functional theory (DFT) used to model and predict molecular properties.
-
Potential applications in materials science: While still under investigation, noble gas compounds, including those of xenon, hold potential applications in materials science for their unique optical and electronic properties.
-
Understanding atmospheric processes: Trace amounts of noble gas compounds can be found in the Earth’s atmosphere. Studying these compounds can help us better understand certain atmospheric processes.
Frequently Asked Questions (FAQ)
-
Q: Is XeCl₂ stable? A: XeCl₂ is relatively unstable at room temperature and decomposes readily. It needs to be synthesized and stored under specific conditions to maintain its integrity.
-
Q: How is XeCl₂ synthesized? A: XeCl₂ is typically synthesized using a low-temperature matrix isolation technique where xenon and chlorine are reacted in an inert gas matrix at very low temperatures.
-
Q: What are some other noble gas compounds? A: Besides XeCl₂, other known noble gas compounds include XeF₂, XeF₄, XeF₆, KrF₂, and various xenon oxides and oxofluorides.
-
Q: Why can xenon form compounds but not helium or neon? A: Helium and neon have much smaller atomic radii and higher ionization energies compared to xenon. Their valence electrons are held more tightly, making it significantly harder to overcome the energy barrier needed to form chemical bonds. Xenon, being a larger and less electronegative atom, allows for easier participation in bonding, particularly with highly electronegative elements like fluorine and chlorine.
-
Q: Can other elements form expanded octets? A: Yes, other elements beyond the third period in the periodic table, particularly those in the p-block, can form expanded octets. This is especially true for elements in higher periods that possess available d orbitals which can accommodate additional electrons.
Conclusion: XeCl₂ – A Testament to the Expanding Frontiers of Chemistry
The Lewis dot structure of XeCl₂, while seemingly simple at first glance, reveals profound insights into the complexities of chemical bonding. This compound serves as a powerful example of how the seemingly rigid rules of chemistry can be expanded to accommodate new discoveries and understanding. The ability of xenon, a noble gas once considered unreactive, to form compounds like XeCl₂ highlights the dynamic nature of scientific knowledge and underscores the ongoing exploration of the chemical world. The study of XeCl₂ and other noble gas compounds not only enhances our fundamental understanding of chemical bonding but also inspires further investigation into the potential applications of these unique molecules in various fields of science and technology.
Latest Posts
Latest Posts
-
16 Oz Is A Lb
Sep 15, 2025
-
What Is 2 5 Equivalent To
Sep 15, 2025
-
90 Confidence Interval Z Score
Sep 15, 2025
-
How Do You Spell Vicious
Sep 15, 2025
-
Structure And Plot Quick Check
Sep 15, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure For Xecl2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.