Molar Mass Of Cuso4 5h2o
cibeltiagestion
Sep 08, 2025 · 6 min read
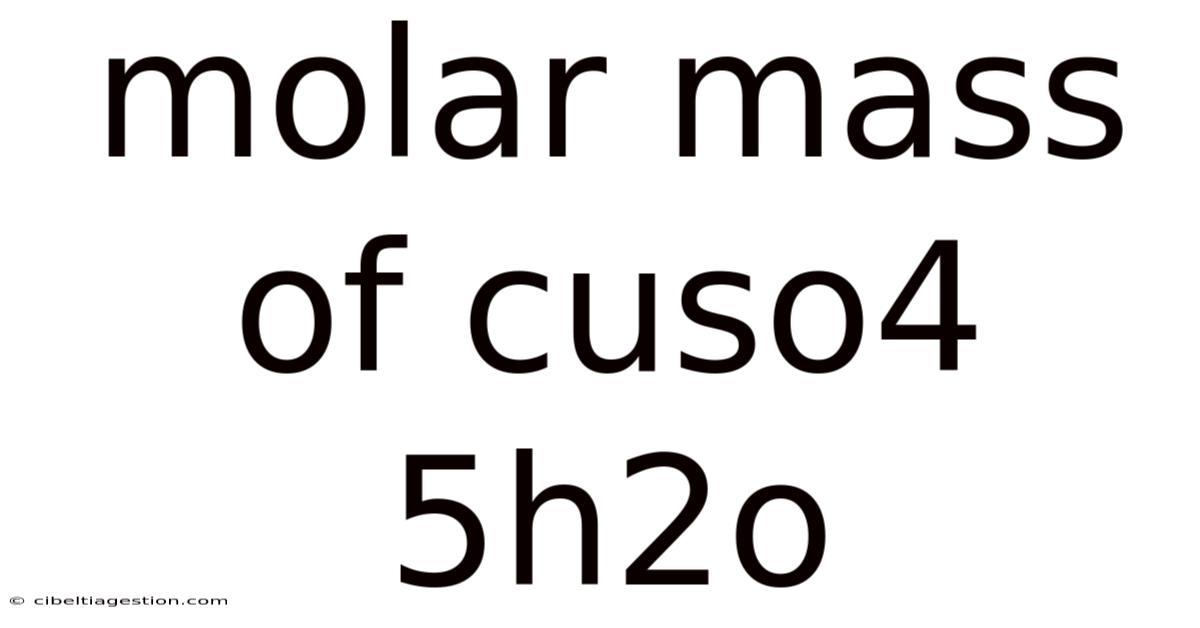
Table of Contents
Understanding the Molar Mass of CuSO₄·5H₂O: A Deep Dive
Determining the molar mass of a compound is a fundamental skill in chemistry, crucial for various calculations including stoichiometry, solution preparation, and more. This article delves into the specific case of copper(II) sulfate pentahydrate (CuSO₄·5H₂O), explaining not just how to calculate its molar mass but also why the process is important and the underlying chemical principles involved. We'll break down the calculation step-by-step, address frequently asked questions, and explore the significance of understanding hydrated salts.
Introduction to Molar Mass and Hydrated Salts
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). A mole is a unit representing Avogadro's number (approximately 6.022 x 10²³) of particles, whether atoms, molecules, or formula units. Calculating molar mass involves summing the atomic masses of all atoms present in a chemical formula.
Copper(II) sulfate pentahydrate (CuSO₄·5H₂O), also known as blue vitriol, is a hydrated salt. This means it contains water molecules incorporated into its crystal structure. The "·5H₂O" indicates that five water molecules are associated with each formula unit of copper(II) sulfate (CuSO₄). These water molecules are not simply adsorbed onto the surface but are chemically bound within the crystal lattice. Understanding this hydration is critical for accurately determining the molar mass.
Step-by-Step Calculation of the Molar Mass of CuSO₄·5H₂O
To calculate the molar mass of CuSO₄·5H₂O, we need the atomic masses of each element present: copper (Cu), sulfur (S), oxygen (O), and hydrogen (H). These values are typically found on the periodic table. For this calculation, let's use the following approximate atomic masses:
- Cu: 63.55 g/mol
- S: 32.07 g/mol
- O: 16.00 g/mol
- H: 1.01 g/mol
Now let's break down the calculation:
-
Molar mass of CuSO₄:
- Cu: 1 atom x 63.55 g/mol = 63.55 g/mol
- S: 1 atom x 32.07 g/mol = 32.07 g/mol
- O: 4 atoms x 16.00 g/mol = 64.00 g/mol
- Total molar mass of CuSO₄ = 63.55 + 32.07 + 64.00 = 159.62 g/mol
-
Molar mass of 5H₂O:
- H: 10 atoms (5 molecules x 2 atoms/molecule) x 1.01 g/mol = 10.10 g/mol
- O: 5 atoms x 16.00 g/mol = 80.00 g/mol
- Total molar mass of 5H₂O = 10.10 + 80.00 = 90.10 g/mol
-
Total molar mass of CuSO₄·5H₂O:
- Total molar mass = Molar mass of CuSO₄ + Molar mass of 5H₂O = 159.62 g/mol + 90.10 g/mol = 249.72 g/mol
Therefore, the molar mass of CuSO₄·5H₂O is approximately 249.72 g/mol. Slight variations may occur depending on the atomic mass values used from different periodic tables.
The Importance of Accurate Molar Mass Determination
Accurately determining the molar mass of CuSO₄·5H₂O, or any compound, is crucial for several reasons:
-
Stoichiometric Calculations: Molar mass is essential for converting between mass and moles in chemical reactions. This allows for accurate predictions of reactant amounts, product yields, and limiting reagents.
-
Solution Preparation: When preparing solutions of a specific concentration (e.g., molarity), the molar mass is used to calculate the required mass of solute to achieve the desired concentration. An inaccurate molar mass will result in an incorrectly prepared solution.
-
Analytical Chemistry: In many analytical techniques, the molar mass is needed to determine the amount of substance present in a sample. This is crucial for various applications, including environmental monitoring, pharmaceutical analysis, and materials science.
-
Understanding Hydrated Salts: The inclusion of water molecules in the molar mass calculation highlights the importance of considering the specific form of a compound. Using the anhydrous molar mass (CuSO₄ only) for calculations involving the pentahydrate would lead to significant errors.
Further Applications and Considerations
The molar mass of CuSO₄·5H₂O has implications beyond simple calculations:
-
Dehydration: Heating CuSO₄·5H₂O drives off the water molecules, resulting in anhydrous copper(II) sulfate (CuSO₄). This process demonstrates the nature of hydrated salts and is often used in experiments to illustrate water of crystallization. The difference in mass before and after dehydration confirms the presence and amount of water molecules.
-
Crystallography: The structure and bonding within the CuSO₄·5H₂O crystal lattice influence its properties, including its solubility and color. Understanding the molar mass is a starting point for exploring these more advanced concepts.
-
Industrial Applications: CuSO₄·5H₂O has numerous industrial applications, including in agriculture (as a fungicide and pesticide), in the textile industry (as a mordant), and in water treatment. Precise molar mass calculations are vital for consistent and effective use in these applications.
Frequently Asked Questions (FAQ)
Q: Why is it important to consider the water molecules in CuSO₄·5H₂O when calculating the molar mass?
A: Ignoring the water molecules would lead to a significantly under-estimated molar mass, resulting in incorrect calculations in any application where the molar mass is used. The water molecules are an integral part of the compound's chemical formula and its properties.
Q: What happens if I use different atomic masses from the periodic table?
A: Slight variations in the final molar mass will occur due to the different precision levels of atomic masses reported on different periodic tables. However, these variations are typically minimal and won't significantly impact most calculations.
Q: Can the molar mass of CuSO₄·5H₂O be experimentally determined?
A: Yes, the molar mass can be experimentally determined through techniques like titration or gravimetric analysis. These methods provide an independent verification of the calculated value.
Q: What are some common errors in calculating molar mass?
A: Common errors include misinterpreting the chemical formula, using incorrect atomic masses, and forgetting to account for the presence of water molecules in hydrated salts. Careful attention to detail is crucial.
Q: How does the molar mass relate to other chemical concepts, such as Avogadro's number?
A: Avogadro's number defines the number of particles in one mole. The molar mass provides the mass of that one mole of particles. Therefore, the molar mass connects the macroscopic (mass) and microscopic (number of particles) scales.
Conclusion
Calculating the molar mass of CuSO₄·5H₂O is a straightforward process that underscores the importance of understanding chemical formulas and the implications of hydration in salts. The accurate determination of molar mass is fundamental to various chemical calculations and applications. This detailed explanation, coupled with the step-by-step guide, should equip you with the necessary knowledge and confidence to perform similar calculations for other compounds. Remember that precision and attention to detail are crucial for obtaining accurate results in all aspects of chemistry.
Latest Posts
Latest Posts
-
One State That Borders Canada
Sep 08, 2025
-
1 Lb Is 16 Oz
Sep 08, 2025
-
Which Sentence States Delucas Claim
Sep 08, 2025
-
1 1 Independent Practice Answer Key
Sep 08, 2025
-
Write Your Research Question Below
Sep 08, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Cuso4 5h2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.