Symbol Of Silver In Chemistry
cibeltiagestion
Sep 04, 2025 · 6 min read
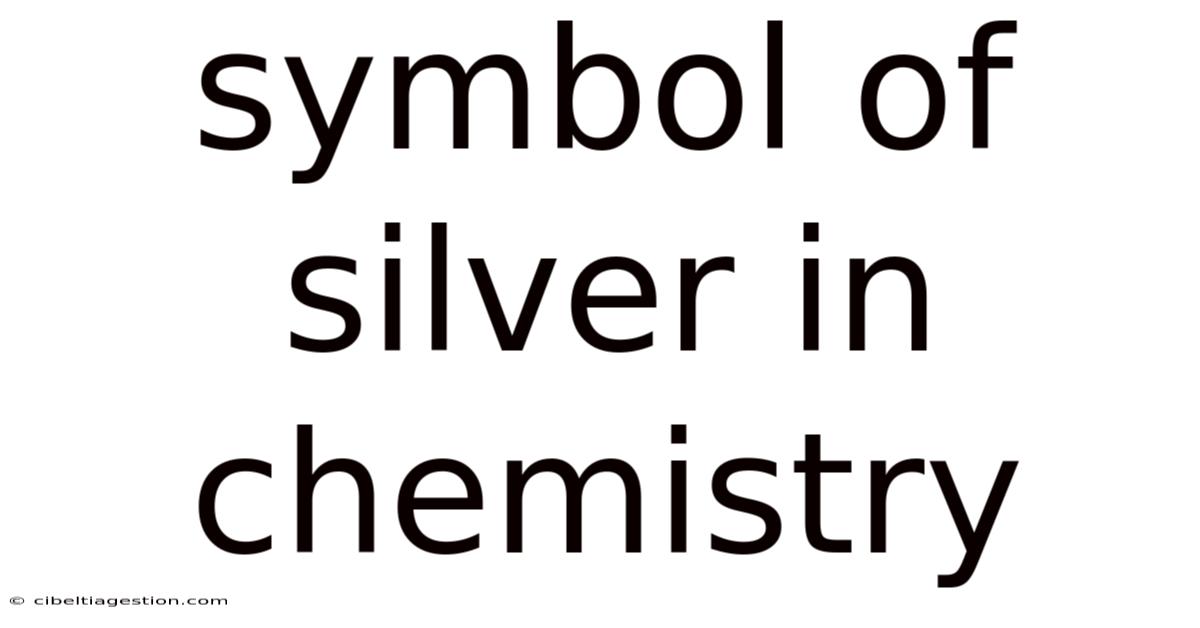
Table of Contents
The Silver Symbol in Chemistry: Exploring Ag and its Significance
The symbol Ag, representing silver in the periodic table, is more than just a shorthand notation for a shiny, precious metal. It unlocks a world of chemical properties, reactions, and applications that have shaped human history and continue to drive innovation in various fields. This article delves deep into the chemical symbolism of silver, exploring its historical context, atomic structure, chemical behavior, common compounds, industrial uses, and its unique role in chemistry and beyond.
A Journey Through History: The Origins of Ag
The symbol Ag, derived from the Latin word "argentum," speaks volumes about silver's historical significance. Ancient civilizations prized silver for its beauty, malleability, and antimicrobial properties. Its use extended beyond adornment; it played crucial roles in coinage, trade, and even early medicine. The choice of "argentum" as the Latin root, and consequently the symbol Ag, highlights silver's enduring value and its place in the development of human societies. The enduring legacy of "argentum" underscores the continued importance of silver in various aspects of modern life.
Unveiling the Atom: Atomic Structure and Properties of Silver
Chemically, silver (Ag) is a transition metal located in Group 11, Period 5 of the periodic table. Its atomic number is 47, meaning it possesses 47 protons in its nucleus. The electronic configuration of silver is [Kr] 4d¹⁰ 5s¹, a configuration that profoundly influences its chemical behavior. This unique electronic arrangement contributes to silver's relatively low reactivity compared to other transition metals, a property crucial to its widespread applications.
The noble nature of silver stems from its filled d-orbital (4d¹⁰). This filled subshell creates a stable electronic configuration, making it less inclined to readily lose or share electrons, hence exhibiting lower reactivity than expected for a transition metal. This relative inertness makes it resistant to corrosion and oxidation under normal atmospheric conditions. However, it can still react under specific conditions, as we will explore further.
Chemical Reactions and Compounds: Exploring Silver's Reactivity
While less reactive than many other metals, silver does participate in chemical reactions. Its oxidation state is predominantly +1, although +2 and +3 oxidation states are also possible, albeit less common and often under specific reaction conditions.
-
Reactions with Halogens: Silver readily reacts with halogens (fluorine, chlorine, bromine, iodine) to form silver halides (AgF, AgCl, AgBr, AgI). These reactions are often used in photographic processes and other chemical applications. For example, the formation of silver chloride (AgCl) as a white precipitate upon adding chloride ions to a silver nitrate solution is a classic qualitative analytical test.
-
Reactions with Acids: Silver is generally unreactive with most dilute acids, further showcasing its noble character. However, oxidizing acids like concentrated nitric acid (HNO₃) can oxidize silver, forming silver nitrate (AgNO₃) and releasing nitrogen dioxide (NO₂). This reaction demonstrates the conditions under which silver's reactivity can be enhanced.
-
Complex Formation: Silver exhibits a strong tendency to form coordination complexes with ligands containing nitrogen, sulfur, or phosphorus atoms. These complexes often display unique properties and are crucial in various catalytic processes and applications. Examples include complexes with ammonia (e.g., [Ag(NH₃)₂]⁺) and thiosulfate (e.g., [Ag(S₂O₃)₂]³⁻) which are relevant in photography and other silver-related industries.
Key Silver Compounds and Their Applications
Several silver compounds play pivotal roles in various industries and scientific applications. Some notable examples include:
-
Silver Nitrate (AgNO₃): This compound is perhaps the most widely used silver compound. It's employed in medicine as an antiseptic and cauterizing agent. It's also used in the production of other silver compounds, as a reagent in analytical chemistry, and in the manufacturing of silver mirrors.
-
Silver Halides (AgCl, AgBr, AgI): These are essential components of photographic films and papers. Their sensitivity to light allows for the formation of latent images which are then developed into visible photographs. The varying sensitivities of these halides to different wavelengths of light contribute to the nuanced capabilities of photographic processes.
-
Silver Sulfadiazine (AgSD): A topical antimicrobial agent used to prevent and treat infections in burns and wounds. This compound leverages silver's intrinsic antimicrobial properties for medicinal purposes.
-
Silver Oxide (Ag₂O): Used in the production of silver batteries, and also finds applications as a catalyst and in certain electronic components.
Industrial Uses of Silver: A Multifaceted Metal
Silver's unique combination of properties renders it invaluable in diverse industries:
-
Electronics: Silver's high electrical and thermal conductivity makes it crucial in electronic components, including printed circuit boards, electrical contacts, and conductive inks. Its use in these applications is driven by the need for reliable and efficient electronic systems.
-
Photography: As mentioned earlier, silver halides are fundamental to traditional photography. Although digital photography is prevalent, certain niche applications still rely heavily on silver-based photographic processes.
-
Catalysis: Silver catalysts play a vital role in various chemical reactions, including the oxidation of ethylene to ethylene oxide, a crucial intermediate in the production of many polymers.
-
Medicine: Silver's antimicrobial properties are exploited in various medical applications, from wound dressings to catheters and other medical devices. This broad application stems from its effectiveness against a wide range of bacteria and its relative biocompatibility.
-
Jewelry and Ornamentation: Silver's lustrous beauty and malleability have made it a cherished material for jewelry and decorative items throughout history. This application showcases the enduring appeal of silver as a precious metal.
FAQ: Addressing Common Questions about Silver in Chemistry
Q: Is silver a toxic element?
A: While silver itself is relatively non-toxic, some of its compounds, particularly soluble silver salts, can exhibit toxicity at high concentrations. It's important to handle silver compounds with appropriate safety measures.
Q: What is the difference between sterling silver and pure silver?
A: Pure silver (99.9% or higher) is soft and easily tarnished. Sterling silver is an alloy containing 92.5% silver and 7.5% other metals, usually copper, which increases its strength and durability while maintaining its lustrous appearance.
Q: How is silver extracted from its ores?
A: Silver extraction involves various methods, including leaching with cyanide solutions and electrolytic refining, depending on the nature of the silver ore. These processes are complex and require specialized knowledge and equipment.
Q: Why is silver used in mirrors?
A: Silver's ability to reflect light effectively makes it ideal for coating the back of glass to create mirrors. This high reflectivity is crucial for producing clear and accurate reflections.
Conclusion: The Enduring Importance of Silver in Chemistry
The silver symbol, Ag, represents far more than just an element on the periodic table. It embodies a rich history, a fascinating chemistry, and a wide range of crucial applications. From its historical significance in coinage to its modern role in electronics and medicine, silver's unique properties have continued to shape our world. Understanding the chemical behavior of silver, its compounds, and its diverse uses is essential not only for chemists but also for anyone interested in the intersection of science, technology, and human history. The exploration of Ag in chemistry continues to yield new discoveries and innovations, ensuring its enduring importance in the years to come. Its role as a catalyst, an antimicrobial agent, and a conductor showcases its versatility and underlines its continued relevance across multiple scientific disciplines. The journey from "argentum" to Ag underscores the evolution of scientific understanding and the enduring impact of this remarkable element.
Latest Posts
Latest Posts
-
If You Find Yourself Hydroplaning
Sep 06, 2025
-
Pre Lab Study Questions 18
Sep 06, 2025
-
He Eats Apples In Spanish
Sep 06, 2025
-
Which Is True Regarding Multitasking
Sep 06, 2025
-
What Is 30 Of 3000
Sep 06, 2025
Related Post
Thank you for visiting our website which covers about Symbol Of Silver In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.