1s2 2s2 2p6 3s2 3p4
cibeltiagestion
Sep 13, 2025 · 7 min read
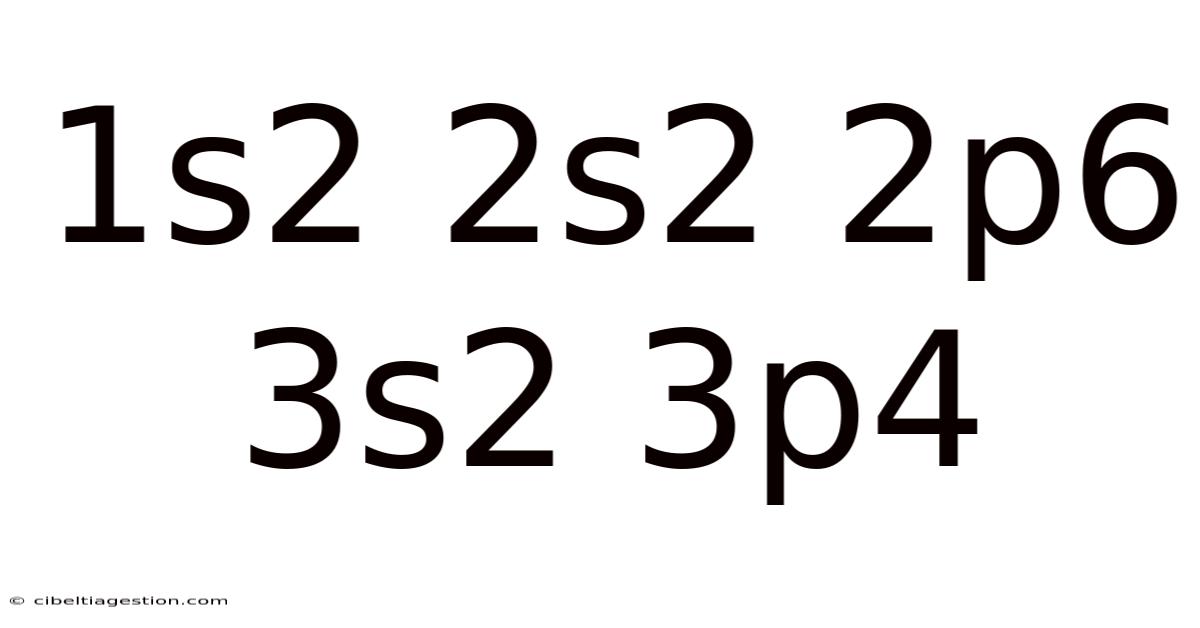
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁴: A Deep Dive into Sulfur's Electronic Structure
This article delves into the fascinating world of electron configuration, specifically focusing on the notation "1s² 2s² 2p⁶ 3s² 3p⁴". This seemingly cryptic sequence reveals the precise arrangement of electrons within a sulfur atom, a fundamental concept in chemistry with far-reaching implications in understanding its properties and behavior. We will explore what this notation means, how it's determined, and what it tells us about sulfur's reactivity and place in the periodic table. Understanding electron configuration is key to unlocking the secrets of atomic structure and chemical bonding.
Introduction: Unveiling the Secrets of Electron Configuration
The notation "1s² 2s² 2p⁶ 3s² 3p⁴" represents the electron configuration of a sulfur atom (S). Electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. It's a fundamental concept that explains an element's chemical properties, its reactivity, and its position within the periodic table. This configuration follows the Aufbau principle, which dictates that electrons fill lower energy levels before higher ones, and Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up.
Each part of the notation holds significant meaning:
-
The number (1, 2, 3): Represents the principal quantum number (n), indicating the energy level or shell. The higher the number, the greater the energy and distance from the nucleus.
-
The letter (s, p): Represents the azimuthal quantum number (l), indicating the subshell. 's' represents a spherical subshell, while 'p' represents a dumbbell-shaped subshell. Other subshells exist (d, f), but are not involved in sulfur's configuration.
-
The superscript (², ⁶, ⁴): Represents the number of electrons in that particular subshell. This number is always less than or equal to the maximum capacity of the subshell (2 for s, 6 for p, 10 for d, 14 for f).
Step-by-Step Construction of Sulfur's Electron Configuration
Let's build sulfur's electron configuration step-by-step to fully grasp its meaning. Sulfur has an atomic number of 16, meaning it has 16 protons and 16 electrons in a neutral atom. We will fill the orbitals according to the Aufbau principle and Hund's rule:
-
1s²: The first energy level (n=1) has only one subshell, the 's' subshell, which can hold a maximum of two electrons. These two electrons fill the 1s orbital.
-
2s²: The second energy level (n=2) also has an 's' subshell, which can hold two electrons. These two electrons fill the 2s orbital.
-
2p⁶: The second energy level also contains a 'p' subshell, which can hold up to six electrons (two electrons in each of the three 2p orbitals – 2p<sub>x</sub>, 2p<sub>y</sub>, and 2p<sub>z</sub>). These six electrons completely fill the 2p subshell.
-
3s²: The third energy level (n=3) starts with an 's' subshell, which again holds two electrons. These fill the 3s orbital.
-
3p⁴: Finally, we reach the 3p subshell, which can hold six electrons. However, sulfur only has four more electrons to accommodate. According to Hund's rule, these four electrons will individually occupy the three 3p orbitals before pairing up. This leaves one 3p orbital empty.
Visualizing Sulfur's Electron Configuration: Orbital Diagrams
To better visualize the electron configuration, we can use orbital diagrams. These diagrams show each orbital as a box, and electrons are represented by arrows. Upward-pointing arrows represent electrons with spin +1/2, and downward-pointing arrows represent electrons with spin -1/2.
For sulfur:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑↓
- 3p: ↑ ↑ ↑ ↓
This diagram clearly shows the four unpaired electrons in the 3p subshell, which are crucial for understanding sulfur's chemical behavior.
The Significance of Sulfur's 3p⁴ Configuration: Chemical Properties and Reactivity
The 3p⁴ configuration is key to understanding sulfur's chemical properties. The presence of four electrons in the 3p subshell, with two unpaired electrons, means sulfur readily forms covalent bonds to achieve a stable octet (eight electrons in its valence shell). This explains why sulfur frequently forms compounds with other elements, particularly those that can provide electrons to complete its octet. Sulfur's reactivity is moderate; it is not as reactive as the halogens but more reactive than phosphorus.
Some examples of sulfur's chemical behavior due to its 3p⁴ configuration include:
-
Formation of sulfides: Sulfur readily reacts with metals to form sulfides (e.g., iron sulfide, FeS). This involves sulfur gaining two electrons to achieve a stable octet, forming a sulfide anion (S²⁻).
-
Formation of oxides: Sulfur reacts with oxygen to form various oxides, such as sulfur dioxide (SO₂) and sulfur trioxide (SO₃). These reactions involve covalent bond formation, sharing electrons to achieve stable octets.
-
Formation of organic sulfur compounds: Sulfur is found in many organic molecules, such as thiols (containing –SH groups) and sulfides (containing –S– groups).
Sulfur's Position in the Periodic Table and its Relationship to Electron Configuration
Sulfur's position in the periodic table is directly related to its electron configuration. Sulfur is located in Group 16 (also known as the chalcogens or oxygen group) and Period 3.
-
Group 16: All elements in Group 16 have six valence electrons (the electrons in the outermost shell), resulting in a similar ns²np⁴ configuration. This shared electron configuration leads to similar chemical properties, such as the tendency to form -2 ions or share electrons to achieve a stable octet.
-
Period 3: The '3' in the electron configuration indicates that the outermost electrons are in the third energy level. This determines the atomic size and other physical properties of sulfur.
Frequently Asked Questions (FAQ)
Q1: What is the difference between the electron configuration and the orbital diagram?
A1: The electron configuration provides a concise notation of the electron distribution (e.g., 1s² 2s² 2p⁶ 3s² 3p⁴). The orbital diagram visually represents the electrons within each orbital, including their spin.
Q2: Can sulfur have a different electron configuration?
A2: In its ground state (lowest energy state), sulfur has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴. However, sulfur can exist in excited states, where one or more electrons are promoted to higher energy levels. These excited states are involved in certain chemical reactions and spectroscopic observations.
Q3: How does the electron configuration relate to sulfur's physical properties?
A3: The electron configuration affects various physical properties. The number of valence electrons influences its bonding capabilities and reactivity. The energy levels of electrons contribute to its ionization energy (energy required to remove an electron) and its atomic radius (size of the atom).
Q4: How is electron configuration determined experimentally?
A4: Electron configuration is not directly observed experimentally but is deduced from various spectroscopic techniques, such as photoelectron spectroscopy (PES). PES measures the energy required to remove electrons from an atom, revealing information about their energy levels and arrangement.
Q5: What are some real-world applications of understanding sulfur's electronic structure?
A5: Understanding sulfur's electron configuration is crucial in various applications. It helps in predicting the reactivity of sulfur in industrial processes (e.g., production of sulfuric acid), designing materials with specific properties (e.g., semiconductors), and understanding the role of sulfur in biological systems (e.g., proteins containing sulfur-containing amino acids).
Conclusion: The Power of Understanding Electronic Structure
The seemingly simple notation "1s² 2s² 2p⁶ 3s² 3p⁴" encapsulates a wealth of information about sulfur's atomic structure and its chemical behavior. By understanding electron configuration, we can predict reactivity, explain bonding patterns, and appreciate the intricate relationship between an element's electronic structure and its place in the periodic table. This knowledge is fundamental to chemistry and has far-reaching applications in various scientific fields. Further exploration of advanced concepts like quantum mechanics and molecular orbital theory provides a deeper understanding of the forces that govern atomic and molecular interactions. This detailed understanding of sulfur's electron configuration underscores the power and elegance of the periodic table and its ability to organize and predict the behavior of matter.
Latest Posts
Latest Posts
-
What Is A Physical Change
Sep 13, 2025
-
What Is A Functional Region
Sep 13, 2025
-
40 Fl Oz To Ml
Sep 13, 2025
-
Gcf Of 12 And 8
Sep 13, 2025
-
38 Degrees F To C
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.