C3h5 No3 3 Molar Mass
cibeltiagestion
Sep 10, 2025 · 5 min read
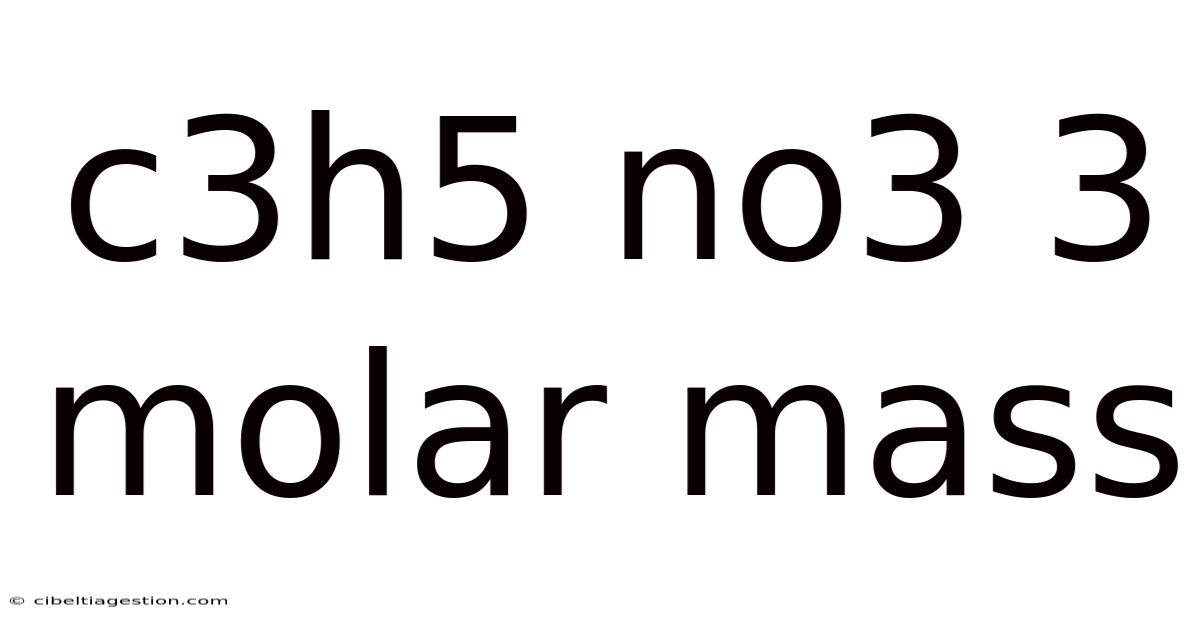
Table of Contents
Understanding the Molar Mass of C3H5(NO3)3: Nitroglycerin
Nitroglycerin, with the chemical formula C₃H₅(NO₃)₃, is a well-known and potent explosive. Understanding its molar mass is crucial in various applications, from its synthesis and handling to its use in medicine (as a vasodilator) and its historical significance in the development of dynamite. This article will delve deep into calculating and understanding the molar mass of C₃H₅(NO₃)₃, exploring the underlying principles and practical implications.
Introduction: What is Molar Mass?
Before we dive into calculating the molar mass of nitroglycerin, let's establish a fundamental understanding of the concept. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). Therefore, the molar mass tells us the mass of 6.022 x 10²³ molecules of a given compound. It is typically expressed in grams per mole (g/mol). Knowing the molar mass is essential for various stoichiometric calculations in chemistry and chemical engineering.
Calculating the Molar Mass of C3H5(NO3)3: A Step-by-Step Guide
Calculating the molar mass of any compound involves summing the atomic masses of all the atoms present in its chemical formula. Let's break down the process for nitroglycerin:
-
Identify the elements present: C₃H₅(NO₃)₃ contains three elements: Carbon (C), Hydrogen (H), Nitrogen (N), and Oxygen (O).
-
Determine the number of atoms of each element: From the formula, we can see that:
- There are 3 Carbon atoms.
- There are 5 Hydrogen atoms.
- There are 3 Nitrogen atoms.
- There are 9 Oxygen atoms (3 from each nitrate group).
-
Find the atomic mass of each element: We use the standard atomic masses from the periodic table:
- Carbon (C): 12.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Nitrogen (N): 14.01 g/mol
- Oxygen (O): 16.00 g/mol
-
Calculate the total mass contribution of each element:
- Carbon: 3 atoms * 12.01 g/mol/atom = 36.03 g/mol
- Hydrogen: 5 atoms * 1.01 g/mol/atom = 5.05 g/mol
- Nitrogen: 3 atoms * 14.01 g/mol/atom = 42.03 g/mol
- Oxygen: 9 atoms * 16.00 g/mol/atom = 144.00 g/mol
-
Sum the individual contributions: Add the mass contributions of each element to obtain the molar mass of nitroglycerin:
36.03 g/mol + 5.05 g/mol + 42.03 g/mol + 144.00 g/mol = 227.11 g/mol
Therefore, the molar mass of C₃H₅(NO₃)₃ (nitroglycerin) is approximately 227.11 g/mol.
Importance of Accurate Molar Mass Determination
The precise determination of molar mass is paramount in several contexts related to nitroglycerin:
-
Synthesis and Production: Accurate molar mass calculations are crucial in determining the stoichiometric amounts of reactants needed for the efficient and safe synthesis of nitroglycerin. Incorrect calculations could lead to incomplete reactions or the formation of unwanted byproducts.
-
Explosive Power and Stability: The molar mass plays a role in understanding the explosive properties of nitroglycerin. The energy released during the detonation is directly related to the molecular composition and mass. Understanding the molar mass is a cornerstone in analyzing and predicting the explosive behavior.
-
Pharmaceutical Applications: In its medical applications as a vasodilator, precise dosage calculations rely heavily on the accurate molar mass of nitroglycerin. The correct molar mass ensures the safe and effective administration of the medication.
-
Environmental Studies: If nitroglycerin is released into the environment, understanding its molar mass is necessary for accurate environmental impact assessments and remediation strategies.
-
Forensic Science and Investigations: In forensic investigations involving explosives, determining the molar mass can help identify and quantify the presence of nitroglycerin at a crime scene.
Beyond the Basics: Isotopes and Molar Mass
While we've used the standard atomic masses in our calculation, it's important to note that elements exist as isotopes. Isotopes are atoms of the same element with different numbers of neutrons and thus different masses. The standard atomic mass reported on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. Therefore, the molar mass we calculated is an average molar mass. For extremely precise calculations, the isotopic composition of the sample needs to be considered.
Frequently Asked Questions (FAQs)
-
Q: Why is the molar mass important in chemistry?
A: Molar mass is a fundamental concept used in many chemical calculations, allowing us to convert between mass and the number of moles of a substance. This is vital for stoichiometry, determining reactant ratios, and understanding reaction yields.
-
Q: Can I use a different method to calculate the molar mass of nitroglycerin?
A: While the step-by-step method is the most straightforward, you could also use molar mass calculators or specialized software designed for chemical calculations. These tools can perform the calculations quickly and accurately, especially for more complex molecules.
-
Q: What are the potential errors in calculating molar mass?
A: Errors can arise from using incorrect atomic masses, miscounting the number of atoms in the chemical formula, or neglecting isotopic variations in the elements. Careful attention to detail is essential for accurate results.
-
Q: What is the practical significance of knowing the molar mass of nitroglycerin?
A: As discussed earlier, the molar mass is crucial for the synthesis, handling, medical applications, environmental assessment, and forensic analysis of nitroglycerin. Its importance spans across multiple scientific and industrial disciplines.
Conclusion: The Significance of Precise Calculations
Understanding and accurately calculating the molar mass of C₃H₅(NO₃)₃, or nitroglycerin, is far more than just a simple arithmetic exercise. It's a fundamental step in numerous applications, from the industrial production of this powerful compound to its careful use in medicine. The precise determination of its molar mass is essential for safety, efficacy, and environmental responsibility. This article aims to provide a comprehensive understanding of this important concept and its real-world implications. By mastering the calculation of molar mass, we gain a deeper appreciation for the fundamental principles of chemistry and its crucial role in various fields of science and technology. The accurate calculation of molar mass isn't just about numbers; it's about ensuring safety, precision, and understanding in the chemical world.
Latest Posts
Latest Posts
-
1 3 Divided By 6
Sep 10, 2025
-
How To Simplify 7 27
Sep 10, 2025
-
Increasing Alcohol Consumption Lowers Gpas
Sep 10, 2025
-
5 X 2 2 125
Sep 10, 2025
-
Ion Expected To Hydrolyze Nacl
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about C3h5 No3 3 Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.