Cr2 So4 3 Nh4 2co3
cibeltiagestion
Sep 07, 2025 · 6 min read
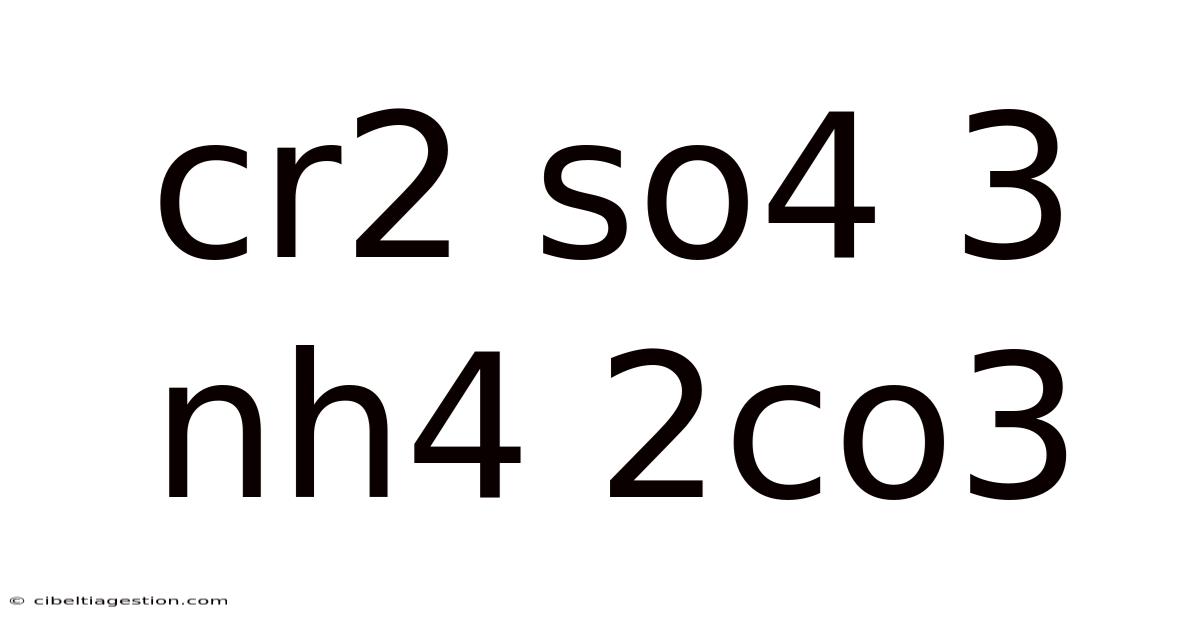
Table of Contents
Delving into the Chemistry of Cr2(SO4)3 and (NH4)2CO3: A Comprehensive Exploration
This article explores the fascinating world of inorganic chemistry, specifically focusing on two important compounds: chromium(III) sulfate (Cr₂(SO₄)₃) and ammonium carbonate ((NH₄)₂CO₃). We will delve into their individual properties, applications, and potential interactions, providing a comprehensive overview suitable for students and anyone interested in learning more about these crucial chemicals. Understanding their characteristics is key to appreciating their roles in various industrial and laboratory settings. This exploration will cover their chemical structures, synthesis methods, reactions, and safety considerations.
Introduction to Chromium(III) Sulfate (Cr₂(SO₄)₃)
Chromium(III) sulfate, also known as chromic sulfate, is an inorganic compound with the chemical formula Cr₂(SO₄)₃. It exists in various forms, including anhydrous and hydrated forms, with the most common being the hydrated form, Cr₂(SO₄)₃·18H₂O. The anhydrous form is a dark-green crystalline solid, while the hydrated forms can vary in color depending on the degree of hydration, often exhibiting shades of violet or green.
Key Properties of Cr₂(SO₄)₃:
- Appearance: Typically appears as a violet or green crystalline solid, depending on the hydration level. The anhydrous form is dark green.
- Solubility: Soluble in water, forming a violet solution that can turn green upon heating due to changes in the chromium(III) complex ions.
- Molar Mass: The molar mass varies depending on the degree of hydration. For the anhydrous form, it's approximately 392.16 g/mol.
- Toxicity: Chromium compounds can be toxic, and Cr₂(SO₄)₃ should be handled with care. Exposure can cause skin irritation, respiratory problems, and other health issues.
- Applications: Cr₂(SO₄)₃ finds applications in various industries, including:
- Leather tanning: Used as a mordant to fix dyes to leather fibers.
- Textile industry: Acts as a mordant in dyeing fabrics, improving colorfastness.
- Catalysis: Used as a catalyst in certain chemical reactions.
- Electroplating: Plays a role in chromium electroplating processes.
- Pigment production: Used in the production of some pigments.
Synthesis of Cr₂(SO₄)₃:
Cr₂(SO₄)₃ can be synthesized through several methods, often involving the reaction of chromium(III) oxide (Cr₂O₃) or chromium(III) hydroxide (Cr(OH)₃) with sulfuric acid (H₂SO₄):
Cr₂O₃ + 3H₂SO₄ → Cr₂(SO₄)₃ + 3H₂O
This reaction typically requires heating to drive the reaction to completion. Other methods involve the oxidation of chromium(II) sulfate or the reduction of chromium(VI) compounds.
Introduction to Ammonium Carbonate ((NH₄)₂CO₃)
Ammonium carbonate, also known as diammonium carbonate, is a white crystalline salt with the chemical formula (NH₄)₂CO₃. It readily decomposes upon heating, releasing ammonia and carbon dioxide. Unlike many other carbonates, it is quite soluble in water.
Key Properties of (NH₄)₂CO₃:
- Appearance: White crystalline solid.
- Solubility: Soluble in water, but its solubility decreases with increased temperature. The solution is slightly alkaline.
- Odor: Possesses a characteristic pungent ammonia odor, especially when exposed to air or heated.
- Decomposition: Readily decomposes upon heating, releasing ammonia (NH₃), carbon dioxide (CO₂), and water (H₂O). This decomposition is responsible for its characteristic odor.
- Molar Mass: Approximately 96.09 g/mol.
- Applications:
- Baking powder: Historically used as a leavening agent in baking, though it has been largely replaced by other, more stable alternatives.
- Fertilizers: Used as a nitrogen source in fertilizers due to its high nitrogen content.
- Pharmaceuticals: Finds some applications in pharmaceutical preparations.
- Textile industry: Plays a role in certain textile processes.
Synthesis of (NH₄)₂CO₃:
Ammonium carbonate can be synthesized by reacting ammonia gas (NH₃) with carbon dioxide (CO₂) in the presence of water:
2NH₃ + CO₂ + H₂O → (NH₄)₂CO₃
This reaction is typically conducted under controlled conditions to maximize yield.
Potential Interactions Between Cr₂(SO₄)₃ and (NH₄)₂CO₃
While not commonly used together in a single application, the interaction between Cr₂(SO₄)₃ and (NH₄)₂CO₃ is theoretically possible. The reaction would likely involve a double displacement reaction (also known as a metathesis reaction), leading to the formation of chromium(III) carbonate and ammonium sulfate:
Cr₂(SO₄)₃(aq) + 3(NH₄)₂CO₃(aq) → Cr₂(CO₃)₃(s) + 3(NH₄)₂SO₄(aq)
However, the chromium(III) carbonate formed, Cr₂(CO₃)₃, is relatively insoluble and would likely precipitate out of the solution. The ammonium sulfate, (NH₄)₂SO₄, would remain dissolved in the solution. The exact nature of the reaction and the resulting precipitate would depend on factors such as concentration, temperature, and pH.
Factors Influencing the Reaction:
- Concentration: Higher concentrations of both reactants would lead to a faster reaction rate and potentially a more complete precipitation of Cr₂(CO₃)₃.
- Temperature: Increasing the temperature might increase the reaction rate, but it could also affect the solubility of the products.
- pH: The pH of the solution would influence the solubility of both the reactants and the products.
- Presence of other ions: The presence of other ions in the solution could interfere with the reaction and affect the yield and purity of the products.
Safety Considerations
Both Cr₂(SO₄)₃ and (NH₄)₂CO₃ present certain safety hazards that require careful handling:
Chromium(III) Sulfate (Cr₂(SO₄)₃):
- Toxicity: Chromium compounds can be toxic if ingested or inhaled. Skin contact can cause irritation. Appropriate personal protective equipment (PPE) should be worn when handling Cr₂(SO₄)₃, including gloves, eye protection, and a lab coat.
- Disposal: Cr₂(SO₄)₃ should be disposed of according to local regulations for hazardous waste.
Ammonium Carbonate ((NH₄)₂CO₃):
- Irritant: Ammonium carbonate can irritate the skin, eyes, and respiratory system. Appropriate PPE should be used during handling.
- Decomposition: The decomposition of ammonium carbonate produces ammonia gas, which is pungent and irritating. Work in a well-ventilated area to avoid inhalation of the gas.
- Disposal: Dispose of ammonium carbonate according to local regulations.
Further Applications and Research
The applications of Cr₂(SO₄)₃ and (NH₄)₂CO₃ extend beyond the ones listed above. Ongoing research continues to explore their potential uses in new areas. For example, Cr₂(SO₄)₃'s role in catalysis is a subject of ongoing investigation, with scientists exploring its effectiveness in various chemical processes. Similarly, the use of ammonium carbonate in controlled release fertilizers is an active area of research.
Frequently Asked Questions (FAQ)
- Q: Is chromium(III) sulfate a strong oxidizing agent? A: No, chromium(III) sulfate is not a strong oxidizing agent. It is more stable in its +3 oxidation state.
- Q: What is the difference between ammonium carbonate and ammonium bicarbonate? A: Ammonium bicarbonate (NH₄HCO₃) is a different compound with a different chemical formula and properties compared to ammonium carbonate ((NH₄)₂CO₃).
- Q: Can ammonium carbonate be used as a primary leavening agent in baking? A: While historically used, its instability makes it unsuitable for most modern baking applications.
- Q: What are the environmental impacts of using chromium(III) sulfate? A: Improper disposal can lead to environmental contamination. Sustainable practices are crucial to minimize any negative environmental impacts.
- Q: Is it safe to mix chromium(III) sulfate and ammonium carbonate without any precautions? A: No, appropriate safety measures, including PPE and working in a well-ventilated area, should always be taken when handling these chemicals.
Conclusion
Chromium(III) sulfate and ammonium carbonate, although distinct in their properties and applications, represent important compounds in various chemical processes. Understanding their individual characteristics, as well as the potential interactions between them, provides a fundamental understanding of their roles in industrial and laboratory settings. Safety precautions are crucial when handling these chemicals due to their potential toxicity and irritant properties. Continued research on their applications promises further advancements in various fields. This comprehensive overview aims to provide a solid foundation for those interested in exploring the rich chemistry of these inorganic compounds. Remember to always consult relevant safety data sheets (SDS) before handling any chemical.
Latest Posts
Latest Posts
-
5x 3 X 4 28
Sep 08, 2025
-
7 X 3 9x 10
Sep 08, 2025
-
12 Fluid Ounces To Ml
Sep 08, 2025
-
Kg In Pounds And Stones
Sep 08, 2025
-
3 Quarts In A Gallon
Sep 08, 2025
Related Post
Thank you for visiting our website which covers about Cr2 So4 3 Nh4 2co3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.